Background: Minimal residual disease (MRD) has emerged as one of the most important prognostic markers in patients with multiple myeloma (MM). However, the prognostic significance of MRD status prior to autologous hematopoietic stem cell transplantation (autoHCT) has not been clearly elucidated.
Methods: In this retrospective single-center study we included adult MM patients who achieved ≥VGPR after induction therapy between 2015-2021, received an upfront autoHCT and had available data for pre-transplant MRD status by next-generation flow cytometry (NGF). The sensitivity of our assay is 1/10 -5 cells (0.001%) based on acquisition and analysis of at least 2 million events. We divided the cohort into pre-transplant MRD negative (MRDneg) and MRD positive (MRDpos) groups. Primary endpoints were progression-free survival (PFS) and overall survival (OS).
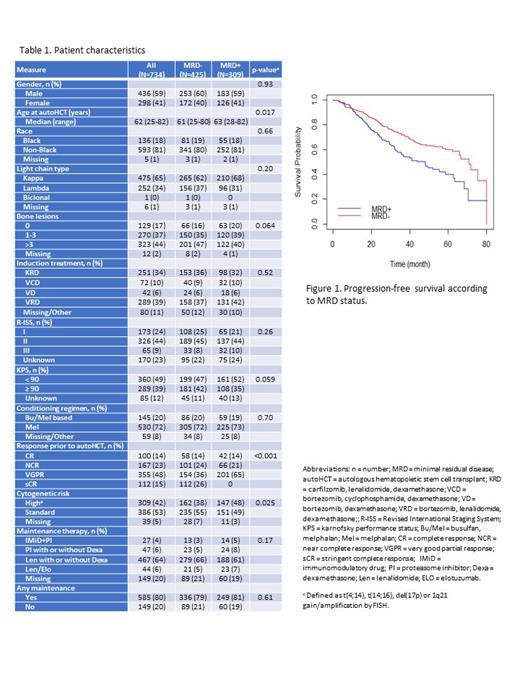
Results: A total of 734 patients were included in our analysis, 425 were MRDneg and 309 were MRDpos at autoHCT. Median age of the entire cohort was 60 (range 25-82) years and 436 (59%) were male. In the MRDpos group, more patients had high-risk cytogenetic abnormalities (48% vs. 38%, respectively; p=0.025), while fewer patients had achieved >CR prior to autoHCT (14% vs. 40%; p<0.001) (Table 1).
At day 100 after autoHCT, 37% of the MRDpos achieved >CR vs. 71% of the MRDneg group, and at best post-transplant response 65% vs. 88% achieved >CR, respectively. At day 100, 16% of the patients in the MRDpos group achieved MRD negative ≥VGPR vs. 27% in the MRDneg group, and at best post-transplant response 33% vs. 37% achieved MRD negative ≥VGPR, respectively.
After a median follow up of 27.6 months (0.7-82.3), the median PFS of the entire cohort was 63.4 months (95% CI 0.3-8.5), and median OS was 80.0 months (95% CI 0.3-82.3). Median PFS was significantly shorter for patients in the MRDpos group compared to MRDneg: 48.2 months (95% CI 0.3-80.5) vs. 80.1 months (95% CI 0.5-80.1), respectively; p<0.001 (Figure 1). There was no significant difference in OS between the two groups (p=0.41).
Pre-transplant MRDpos status (HR 1.80, 95% CI 1.31-2.46; p<0.001) and high-risk cytogenetics (HR 1.86, 95% CI 1.32-2.61; p<0.001) were predictive of shorter PFS in MVA. Use of post-transplant maintenance was predictive of longer PFS (HR 0.49, 95% CI 0.34-0.69; p<0.001) and OS (HR 0.19, 95% CI 0.11-0.32; p<0.001) in MVA.
In a subset analysis, pre-transplant MRD status remained highly predictive of PFS in patients with high-risk cytogenetics (HR 1.48; 95% CI 1.03-2.11; p=0.033) as well as non-high-risk cytogenetics (HR 1.75; 95% CI 1.13-2.70; p=0.012) and in patients with a response of VGPR prior to transplant (HR 1.79; 95% CI 1.33-2.42; p<0.001). Similarly, in patients with R-ISS stage I and II MM, pre-transplant MRD status was highly predictive of PFS [(HR 2.28; 95% CI 1.21-4.29; p=0.011) and (HR 1.73; 95% CI 1.17-2.56; p=0.006), respectively]. However, for patients with R-ISS III disease (HR 2.00; p=0.079), for patients with a response of ≥CR prior to transplant (HR=0.85; p=0.67) and for patients ≥65 years (HR=1.46; p=0.062) the pre-transplant MRD status was not significantly predictive of PFS.
Conclusions: In patients achieving >VGPR to induction, pre-transplant MRDpos status was associated with a lower CR rate after autoHCT and a shorter PFS.
Disclosures
Bashir:Stemline: Research Funding; Acrotech: Research Funding; GSK: Research Funding; Pfizer: Research Funding. Srour:Orca Bio: Research Funding. Saini:Panbela Theraputics: Research Funding; GSK: Research Funding. Lin:Takeda: Patents & Royalties, Research Funding. Nieto:Secura Bio: Research Funding; Astra Zeneca: Research Funding; Affimed: Research Funding. Lee:Amgen: Research Funding; Regeneron: Consultancy, Research Funding; Allogene Thereapeutics: Consultancy; Takeda Pharmaceuticals: Consultancy, Research Funding; Monte Rosa Therapeutics: Consultancy; Pfizer: Consultancy; Sanofi: Consultancy; GlaxoSmithKline: Consultancy, Research Funding; Genentech: Consultancy; Bristol Myers Squibb: Consultancy, Research Funding; Janssen: Consultancy, Research Funding; Celgene: Consultancy. Patel:AbbVie; Allogene Therapeutics, Inc.; Arcellx; Bristol Myers Squibb/Celgene Corporation; Cellectis; Janssen Pharmaceuticals, Inc.; Nektar Therapeutic; Poseida Therapeutics; Precision BioSciences, Inc.; and Takeda Pharmaceuticals U.S.A., Inc.: Research Funding; Takeda: Consultancy; AbbVie; Arcellx, AstraZeneca; Bristol Myers Squibb/Celgene Corporation; Caribou Science; Cellectis; Curio Bioscience; Genentech; Janssen Pharmaceuticals, Inc.; Karyopharm; Legend Biotech; Merck & Co., Inc.; Oncopeptides; Pfizer; Precision BioSciences: Consultancy. Kebriaei:Pfizer: Consultancy, Honoraria; Jazz: Consultancy, Honoraria. Thomas:Bristol Myers Squibb, Janssen Pharma Genentech, X4 pharma, Cellectar Biosciences, Ascentage Pharma: Research Funding; Genentech: Research Funding; X4 pharma: Research Funding; Cellectar Biosciences: Research Funding; Janssen Pharma: Research Funding; Ascentage Pharma: Research Funding; Cellectar Biosciences: Consultancy; Abbvie, Cellectar Biosciences: Consultancy. Orlowski:BMS/Celgene Corporation, CARsgen Therapeutics, Exelixis Inc., Heidelberg Pharma, Janssen Biotech Inc., Sanofi/Genzyme, Takeda Pharmaceuticals USA Inc.: Other: Clinical Research Funding, Research Funding; Asylia Therapeutics, BioTheryX Inc., Heidelberg Pharma: Other: Laboratory Research Funding, Research Funding; Asylia Therapeutics: Current equity holder in private company, Patents & Royalties; AbbVie, Adaptive Biotech, Asylia Therapeutics, Inc., BioTheryX, Bristol-Myers Squibb Pharmaceuticals, Karyopharm Therapeutics, Meridian Therapeutics, Monte Rosa Therapeutics, Nanjing IASO Biotherapeutics, Neoleukin Corporation, Oncopeptides AB, Pfizer, In: Consultancy, Honoraria. Shpall:Celaid Therapeutics: Membership on an entity's Board of Directors or advisory committees; Fibrobiologics: Membership on an entity's Board of Directors or advisory committees; Axio: Membership on an entity's Board of Directors or advisory committees; Affimed: Other: License agreement; Takeda: Other: License agreement; Syena: Other: License agreement; NY Blood Center: Membership on an entity's Board of Directors or advisory committees; Adaptimmune: Membership on an entity's Board of Directors or advisory committees; Navan: Membership on an entity's Board of Directors or advisory committees. Champlin:Orca Bio: Consultancy; Takeda Corporation: Patents & Royalties; Johnson & Johnson/Janssen: Consultancy; Omeros: Consultancy; Kadmon: Consultancy; Arog: Consultancy; Actinium Pharmaceuticals: Consultancy; Cell Source: Research Funding. Qazilbash:Bioline: Other: Advisory board; NexImmune: Research Funding; Amgen: Research Funding; Angiocrine: Research Funding; Janssen: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal